Abstract
Inherited thrombophilia and cancer are both independently known to increase the risk of venous thromboembolism (VTE) due to genetic procoagulant predisposition, tissue damage and inflammatory mediators, respectively. The role of inherited thrombophilia on the risk of VTE in cancer patients is unclear and determination of thrombophilic status is not part of clinical practice. Our objective was to determine the prevalence of common thrombophilia gene mutations and their influence on the risk of developing VTE in a cohort of moderate to high-risk ambulatory cancer patients receiving chemotherapy.
We used buffy coat blood samples at the baseline visit from patients enrolled in the AVERT trial (a randomized, placebo-controlled, double-blind trial to assess the efficacy and safety of apixaban as primary thromboprophylaxis in ambulatory cancer patients with intermediate to high risk for VTE) to determine if a panel of thrombophilia gene mutations (including Prothrombin G20210A mutation, Factor XI, Fibrinogen gamma chain (FGG), SERPINA10, Factor V K858R, Factor XIII, Factor V Leiden and, ABO blood gene mutations) is associated with a diagnosis of VTE during the 7-months follow-up after randomization (which in all cases coincided with the start of chemotherapy). Bivariate tests of association were performed using the Fisher exact test. Logistic regression analysis was used to calculate unadjusted and adjusted odds ratios (OR) with 95% confidence intervals comparing heterozygous and homozygous mutations (combined) to wild type. When a gene mutation was rare or absent in the cohort, we used exact logistic regression to obtain OR estimates. We adjusted for age, sex, anticoagulation treatment group, and concomitant antiplatelet therapy.
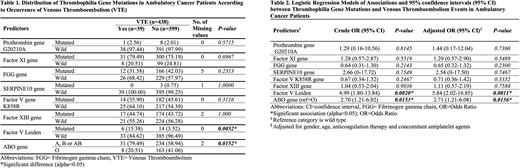
438 patients (260 females) (76.3% of the entire cohort of 574) with available samples were included in this study. The patients' median age was 62. During the 7-months of follow-up, there were 39 VTE events including 25 deep vein thrombosis (DVT), 13 pulmonary embolisms (PE), and 1 DVT with PE. Among the 39 (8.90%) patients who suffered a VTE event, the prevalence of mutations was Prothrombin G20210A mutation: 2.56%, Factor XI: 79.5%, FGG: 31.6%, SERPINA10: 0%, Factor V K858R: 35.9%, Factor XIII: 44.7%, Factor V Leiden: 15.4% and ABO: 79.5%, compared to Prothrombin G20210A mutation: 2.01%, Factor XI: 75.2%, FGG: 42.0%, SERPINE10: 0.75%, Factor V K858R: 45.6%, Factor XIII: 43.7%, Factor V Leiden: 3.52% and ABO: 58.9% in those who did not develop a VTE (n=399, Table 1). The odds of VTE were significantly increased in patients with Factor V Leiden mutation compared to those without the mutation, in both unadjusted and adjusted models [OR: 4.99 (p=0.0020) and adjusted OR: 5.84 (p=0.0011)] (Table 2). Patients with non-O blood type also had increased odds of VTE compared to those with O blood type before and after adjustment [OR: 2.70 (p=0.0153) and adjusted OR: 2.71 (p=0.0156)] (Table 2). No other thrombophilia genes were significantly associated with the occurrence of VTE (Table 2).
In conclusion, our data indicate that some thrombophilia gene mutations may be important predictors for VTE in cancer patients. The Factor V Leiden and ABO gene mutations independently predicted VTE in this cohort of moderate to high-risk ambulatory cancer patients receiving chemotherapy. Whether testing for thrombophilia gene mutations can help risk stratify or influence outcomes should be further explored in prospective studies before clinical application.
Disclosures
Wang:Leo Pharma: Research Funding; Servier: Other: Advisory board; Valeo: Other: Advisory board. Wells:Bayer HealthCare: Honoraria. Carrier:Leo Pharma: Honoraria, Research Funding; BMS: Honoraria, Research Funding; Pfizer: Honoraria, Research Funding; Sanofi: Honoraria; Servier: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal